“[Introduction] Human immunodeficiency virus preexposure prophylaxis (PrEP) with tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) is highly effective in preventing new HIV infections in diverse populations when taken as prescribed. The US Preventive Services Task Force (USPSTF) now provides a grade A recommendation to offer PrEP for individuals at high risk for HIV acquisition. Despite proven efficacy and guideline endorsement, only a minority of the estimated 1.2 million individuals in the United States who are eligible have started using PrEP. General internists and primary care physicians (PCPs) are key to scaling up the use of PrEP as a strategy for ending the HIV epidemic.
Risk Assessment [“yes” to any question indicates PrEP eligibility]
Questions to be framed as part of an open-ended discussion of sexual health
- Do you have condomless anal or vaginal sex with 1 or more sexual partner(s)?
- Do you have condomless anal or vaginal sex, or share injection drugs with 1 or more partner(s) with an unknown HIV status?
- Is your sexual or injection drug partner HIV-infected with viral load unknown or not suppressed?
- Do you engage in transactional sex (ie, sex for money, housing, or drugs)?
- Do you identify as a transgender man or woman who has sex with men?
- Do you inject drugs with nonsterile and/or shared needles?
- Have you been diagnosed with chlamydia or gonorrhea at a nonthroat site or syphilis in the past 6 months?
Note: The initiation of preexposure prophylaxis (PrEP) may be considered on a case-by-case basis for individuals who would like to start PrEP, do not meet the above criteria, and understand the risks/benefits.
If any of the above are answered in the affirmative, proceed to clinical assessment.
Baseline clinical assessment
- Order fourth-generation HIV Ag/Ab (antigen/antibody) laboratory test, assessment of creatinine clearance (CrCl), hepatitis B serologic testing, hepatitis C antibody (HCV Ab), and pregnancy test (if applicable).
- Assess for symptoms consistent with acute HIV; if suggestive signs or symptoms are present, obtain HIV RNA assay results.
- Order sexually transmitted infection (STI) testing (neisseria gonorrheaea and chlamydia trachomatis of throat/urine/rectum/vagina, syphilis).
If HIV uninfected (by above criteria) and CrCl greater than 60 mL/min, proceed to prescribing PrEP.
Prescribing PrEP
- Prescribe daily tenofovir disoproxil fumarate (TDF)/emtricitabine (FTC) (no more than 90-day supply, 1 fixed-dose tablet by mouth daily) or on-demand “2-1-1” TDF/FTC (for men who have sex with men only)—note: the prescription can be initiated without return to clinic once appropriate testing has resulted and has been reviewed.
- Advise patient regarding 7-day lead in time for anal and/or vaginal sex.
- Advise regarding potential adverse effects (gastrointestinal, renal, bone).
- Advise that PrEP does not protect from bacterial STIs and quarterly monitoring is recommended.
- Advise patient to apply for PrEP Assistance Program and review potential assistance and navigation programs available in their state.
- Offer vaccinations for hepatitis A, hepatitis B virus, human papilloma virus and/or meningococcal infections for eligible individuals.
- Reinforce dosing schedule and adherence.
Follow-up
- At 1 month: ambulatory clinical visit for follow-up HIV testing, address potential PrEP-related toxic effects, evaluate and support against potential barriers to adherence.
- Every 3 months: ambulatory clinical visit to repeat HIV testing, STI testing (neisseria gonorrheaea and chlamydia trachomatis of throat/urine/rectum/vagina, syphilis), pregnancy testing (if applicable), evaluate and support against potential barriers to adherence, and refill 90-day prescription.
- Every 6 months: repeat CrCl assessment (quarterly if age >50 years, baseline CrCl <90 mL/min, or diagnoses of diabetes or hypertension).
- Every 12 months: HCV Ab (more frequently if higher risk).
Note: some clinics have leveraged nonprovider support services to perform quarterly visits, mandating provider visits annually, or if challenges arise.”
Preexposure prophylaxis medication and service cost is a frequent and valid concern raised by many patients. Out-of-pocket expenses for TDF/FTC, associated clinical visits, and recommended laboratory testing are highly variable based on insurance coverage. However, given the recent USPSTF grade A recommendation to offer PrEP for individuals at increased risk for HIV, insurers will be obligated to cover the services without cost sharing for patients within 1 year of the recommendation.22 Several programs provide drug copay assistance, with some state programs offering additional clinical visit and lab cost assistance.”
HIV Preexposure Prophylaxis—The Role of Primary Care Clinicians in Ending the HIV Epidemic, JAMA Internal Medicine, 2019.11.18
“[Study Overview] Using the characteristics of 14 946 patients aged 75 years and older with incident AF in the Anticoagulation and Risk Factors in Atrial Fibrillation-Cardiovascular Research Network (ATRIA-CVRN) cohort, we estimated the net clinical benefit (NCB) of anticoagulation by age. We used the Atrial Fibrillation Decision Support Tool, a well-established 29-state Markov decision analytic model, to estimate the NCB of anticoagulation for each patient in the ATRIA-CVRN cohort in quality-adjusted life years (QALYs).
[Results] The estimated NCB of anticoagulation with warfarin and apixaban decreased with age (Figure 1). Among patients aged 75 years, using the CHA2DS2-VASc score to estimate stroke risk, warfarin resulted in a median lifetime NCB of 0.45 QALYs (interquartile range [IQR], 0.25–0.72 QALYs). Among patients aged 87 years, the median lifetime NCB associated with warfarin decreased to 0.10 QALYs (IQR, 0.04–0.16 QALYs), which was not significantly different from the prespecified threshold for minimal NCB of 0.10 QALYs (P=0.28). Among patients aged 75 years eligible for apixaban therapy (eg, without CKD 4+), using the CHA2DS2-VASc score to estimate stroke risk, apixaban resulted in a median lifetime NCB of 0.74 QALYs (IQR, 0.49–1.06 QALYs). Among patients aged 92 years, the median lifetime NCB associated with apixaban decreased to 0.10 QALYs (IQR, 0.07–0.13 QALYs), which was not significantly different from the prespecified threshold for minimal NCB of 0.10 QALYs (P=0.75).
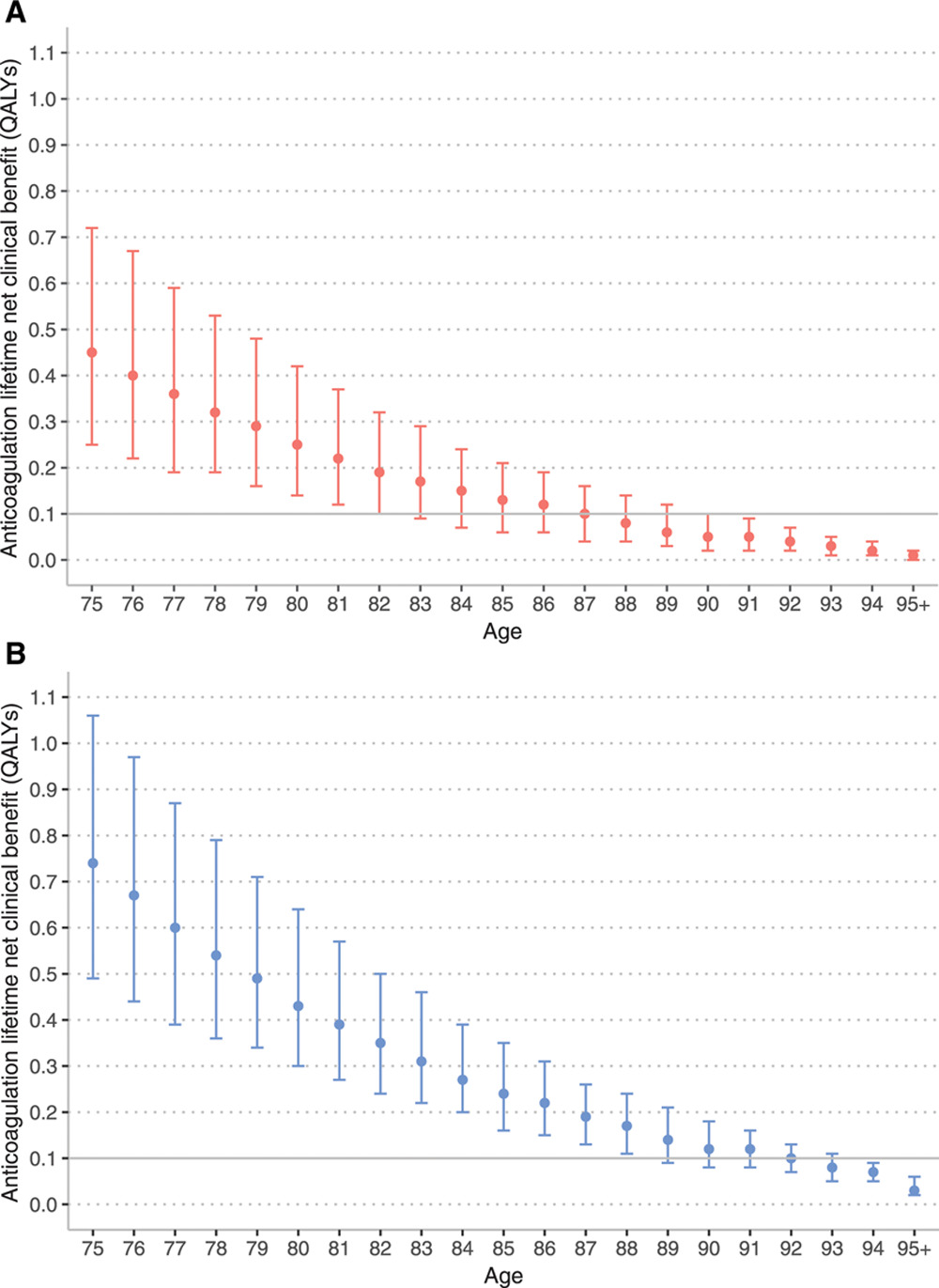
Lifetime net clinical benefit of anticoagulation by age using CHA2DS2-VASc stroke score. A, Warfarin. B, Apixaban. Marker indicates median lifetime net clinical benefit for age, bar indicates interquartile range. Ischemic stroke risk was estimated using the CHA2DS2-VASC score. QALY indicates quality-adjusted life year.
[Discussion] To ground the notion of 0.1 QALYs over a lifetime as minimal benefit, in Table 3 [reproduced below], we list the QALYs gained with selected clinical interventions. For instance, increasing from conventional dose to high-dose statin in patients with stable coronary disease results in a lifetime benefit of 0.1 QALYs per person.
| Selected Interventions | Lifetime QALY Gain |
|---|---|
| Lung cancer screening (age, 55–75 y with 30 pack-year history) | 0.02 |
| High vs conventional dose statin for stable coronary artery disease | 0.10 |
| Aspirin for primary prevention with new diagnosis of diabetes mellitus | 0.19 |
| High vs conventional dose statin following acute coronary syndrome | 0.35 |
[..] While this study finds that the NCB of anticoagulation declines with advancing age, prior observational studies have found the opposite. Prior studies are limited by selection bias (i.e., those treated are more likely to benefit than the untreated) and often do not account for the competing risk of death (i.e., death from non-AF–related causes). The decision analytic model used in this study minimizes selection bias by estimating treatment effects for all patients and intrinsically accounts for the competing risk of death. The results demonstrate that the competing risk of death is an important consideration when estimating the NCB of anticoagulation therapy. We find failing to account for competing risks likely overestimates the NCB of anticoagulation, an effect that is more pronounced at older ages and with more effective anticoagulants.
The results of this study have important implications for the clinical care of older adults with AF. If using the CHA2DS2-VASc stroke risk score, consensus guidelines recommend anticoagulant use for all adults 75 years and older with AF. This study of adults 75 years and older finds that while most are likely to benefit, the NCB decreases every year beyond 75. Further, the results indicate that competing risks (e.g., life-limiting conditions such as end-stage kidney disease) are a major determinant of reduced NCB at a population level. These results, however, must be translated by clinicians to the care of individual patients. For example, the results indicate that for half of 92-year olds with AF, apixaban may not confer a net benefit. Clinicians must translate if an individual patient’s comorbidity burden will limit the benefits of anticoagulants. This information can then inform a shared decision-making encounter so that the anticoagulation decision advances individual patients’ health priorities.
Net Clinical Benefit of Oral Anticoagulation Among Older Adults With Atrial Fibrillation, Circulation: Cardiovascular Quality and Outcomes. 2019.11.11
“[Setting and Participants] Participants included veterans enrolled from Veterans Affairs Medical Center clinics between January 12, 2015, and May 30, 2017. Eligibility was assessed by electronic health record or telephone, followed by in-person screening, at which point written informed consent was obtained. Key eligibility criteria were type 2 diabetes, HbA1c level of at least 8.0% (≥7.5% if age <55 years), body mass index (BMI; calculated as weight in kilograms divided by height in meters squared) of 27 or higher, and interest in losing weight. Key exclusion criteria were (1) age of at least 75 years; (2) hemoglobinopathy; (3) certain chronic or unstable diseases; (4) pregnancy, breastfeeding, or lack of birth control if premenopausal; (5) dementia, unstable psychiatric illness, or substance abuse; and (6) enrollment in another study that might affect the main outcomes. To increase slower than expected enrollment, the following changes were made: (1) removal of HbA1c upper limit of 12.0% and decreased lower limit to at least 7.5% if younger than 55 years, (2) removal of recent weight loss exclusion, (3) shortened exclusion for recent unstable heart disease to 1 month, and (4) addition of the Greenville site.
[Interventions] Participants were assigned to intervention groups (goal of 8-15 participants per group) based on their arm assignment. Sessions for both arms lasted approximately 1.5 to 2 hours and started with outcome data collection by research staff, followed by group counseling led by a registered nurse or registered dietitian. Sessions ended with a one-on-one meeting with a study physician assigned to the group. The study physicians included 2 general internists and 4 endocrinologists. Participants in both arms received a nutrient handbook and similar information emphasizing adequate hydration and physical activity.
Participants in the GMV [group medical visit] groups met every 4 weeks for 16 weeks and every 8 weeks thereafter for diabetes counseling and medication management. The goal of the GMV intervention was to enhance overall diabetes management. A nurse led the class in discussing topics associated with diabetes self-management, such as foot care, managing hypoglycemia, and preventing diabetes complications. After the class, each participant met with a study physician to review blood glucose and hypoglycemic episode data, address any health concerns, and optimize medications for glycemic control and cardiovascular disease prevention using a study-specific algorithm and clinical guidelines.
Participants in the WM/GMV groups met every 2 weeks for 16 weeks for WM [weight management] counseling and medication management and every 8 weeks thereafter for diabetes counseling, medication management, and continued WM support. The WM/GMV intervention included low-carbohydrate nutrition, physical activity, and weight management counseling. A dietitian provided the nutritional counseling using a book and handouts. Initially, carbohydrate intake was restricted to approximately 20 to 30 g per day with no specified caloric restriction. Participants were taught how to add to daily carbohydrate intake gradually as they approached their weight goal or if adherence was threatened. Classes covered topics, such as grocery shopping, restaurant eating, and recipe makeovers, and incorporated behavioral techniques to improve adherence. Physical activity was emphasized at later visits to aid weight maintenance, with discussions on overcoming barriers and demonstrations of home exercises. With use of an algorithm, doses of diabetes medications related to hypoglycemia (insulin and sulfonylureas) and/or weight gain (thiazolidinediones) were reduced by a study physician during the first 16 weeks and optimized for glycemic control thereafter. Because of the diuresis that occurs at diet initiation, low-dose diuretics were discontinued and higher doses were reduced; doses were reinstated at future visits if blood pressure or peripheral edema warranted.

[Discussion] This study compared 2 GMV-based counseling approaches to diabetes management: 1 approach focusing on diabetes medication management for glycemic control (GMV) and the other focusing on intensive WM using a low-carbohydrate diet in addition to diabetes medication management (WM/GMV). Although the patients receiving the WM/GMV approach had greater initial improvement in glycemic control, by 48 weeks, glycemic control converged to the improvement seen with medication management alone. However, the WM/GMV approach led to greater weight loss, reduced diabetes medication use, and fewer hypoglycemic events during 48 weeks. When GMVs are used for diabetes, WM/GMV should be considered as an alternative, noninferior approach for glycemic management that has additional clinical advantages.”
“In this multicentre, controlled, prospective endpoint trial, 19 084 hypertensive patients (10 614 men/8470 women, 60.5 ± 13.7 years of age) were assigned (1:1) to ingest the entire daily dose of ≥1 hypertension medications at bedtime (n = 9552) or all of them upon awakening (n = 9532). At inclusion and at every scheduled clinic visit (at least annually) throughout follow-up, ambulatory blood pressure (ABP) monitoring was performed for 48 h. During the 6.3-year median patient follow-up, 1752 participants experienced the primary CVD outcome (CVD death, myocardial infarction, coronary revascularization, heart failure, or stroke). Patients of the bedtime, compared with the upon-waking, treatment-time regimen showed significantly lower hazard ratio—adjusted for significant influential characteristics of age, sex, type 2 diabetes, chronic kidney disease, smoking, HDL cholesterol, asleep systolic blood pressure (BP) mean, sleep-time relative systolic BP decline, and previous CVD event—of the primary CVD outcome [0.55 (95% CI 0.50–0.61)] and each of its single components (P < 0.001 in all cases), i.e. CVD death [0.44 (0.34–0.56)], myocardial infarction [0.66 (0.52–0.84)], coronary revascularization [0.60 (0.47–0.75)], heart failure [0.58 (0.49–0.70)], and stroke [0.51 (0.41–0.63)].
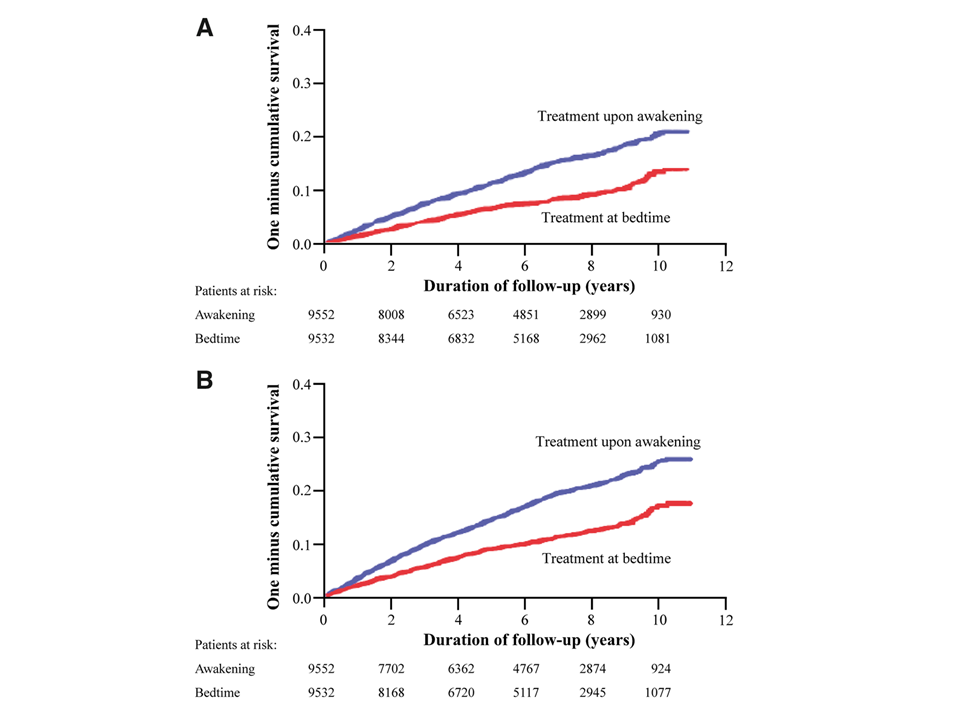
Kaplan–Meier cumulative hazard curves for cardiovascular disease outcome as a function of hypertension treatment-time regimen (either upon awakening or at bedtime). (A) Cardiovascular disease outcome: composite of cardiovascular disease death, myocardial infarction, coronary revascularization, heart failure, and stroke; log-rank: 140.1, P < 0.001. (B) Total cardiovascular disease events: composite of cardiovascular disease death, myocardial infarction, coronary revascularization, heart failure, stroke, angina pectoris, peripheral artery disease, and transient ischaemic attack; log-rank: 174.0, P < 0.001.
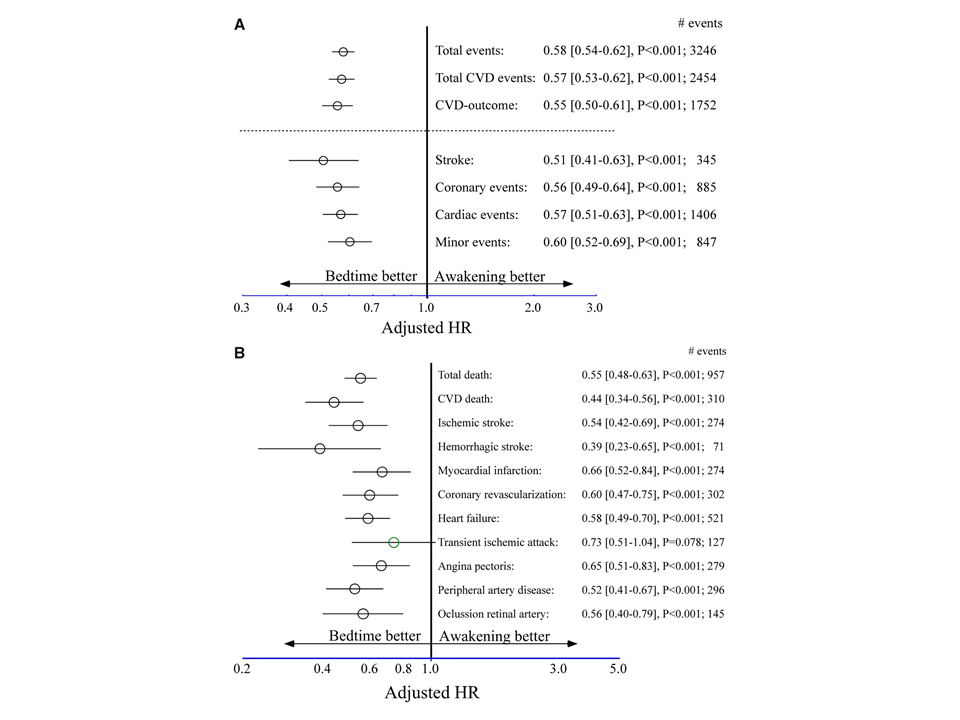
Adjusted hazard ratio of cardiovascular disease outcome as a function of hypertension treatment-time regimen (either upon awakening or at bedtime). (A) Adjusted hazard ratio (95% CI) of primary and secondary composite endpoints. Total events: death from all causes, myocardial infarction, coronary revascularization, heart failure, ischaemic and haemorrhagic stroke, angina pectoris, peripheral artery disease, thrombotic occlusion of the retinal artery, and transient ischaemic attack. Total cardiovascular disease events: cardiovascular disease death, myocardial infarction, coronary revascularization, heart failure, stroke, angina pectoris, peripheral artery disease, and transient ischaemic attack. Cardiovascular disease outcome: cardiovascular disease death, myocardial infarction, coronary revascularization, heart failure, and stroke. Coronary events: cardiovascular disease death, myocardial infarction, and coronary revascularization. Cardiac events: coronary events and heart failure. Minor events: angina pectoris, peripheral artery disease, thrombotic occlusion of the retinal artery, and transient ischaemic attack. (B) Adjusted hazard ratio (95% confidence interval) for each evaluated single endpoint. Adjustments were applied for significant influential baseline characteristics of age, sex, type 2 diabetes, chronic kidney disease, smoking, HDL cholesterol, previous cardiovascular disease event, asleep systolic blood pressure mean, and sleep-time relative systolic blood pressure decline.
Conclusion: Routine ingestion by hypertensive patients of ≥1 prescribed BP-lowering medications at bedtime, as opposed to upon waking, results in improved ABP control (significantly enhanced decrease in asleep BP and increased sleep-time relative BP decline, i.e. BP dipping) and, most importantly, markedly diminished occurrence of major CVD events.”
Bedtime hypertension treatment improves cardiovascular risk reduction: the Hygia Chronotherapy Trial (2019.10.22)