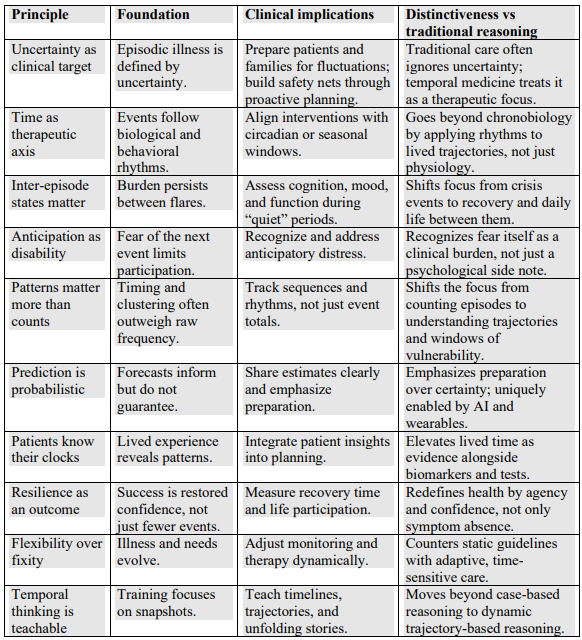
“Although physicians excel at mapping disease spatially (eg, locating tumors, charting lesions, pursuing biomarkers), patients organize their lives around temporal uncertainty. This pattern extends across episodic conditions. Patients often find that uncertainty between episodes causes more distress than the acute symptoms. They organize their lives around the possibility of recurrence, not the reality of crisis.
Chronic episodic disorders do not fit easily into static disease models. Yet clinical coding captures diagnoses, not trajectories. Quality metrics reward event suppression, not functional recovery. A routine office visit might document recent symptoms but overlook emerging vulnerability. In many such conditions, success has been equated with reducing flare-ups. But for patients, quality and success may mean having confidence during the periods between flare-ups.
Temporal medicine is a framework that aims to fill this gap and complements rather than replaces spatial reasoning by shifting the axis of inquiry. Instead of asking only where disease resides, temporal medicine asks when risk is likely to increase, when an intervention is most effective, and how confidence can be restored in the uncertain spaces between episodes. These are not abstract questions and the corresponding responses determine whether patients travel, work, or make long-term plans.
Temporal medicine overlaps with, but is not reducible to, concepts such as disease self-management or personalized medicine. Those approaches emphasize autonomy, education, or genomic tailoring. Chronobiology and chronotherapy highlight circadian and seasonal rhythms but remain largely mechanistic and based on the population. None of these approaches directly address the lived vulnerability that defines episodic illness.
What distinguishes temporal medicine is its explicit framing of vulnerability in time as a clinical target. By naming and systematizing this dimension, temporal medicine shifts what clinicians treat. Traditional reasoning centers on the event (the seizure, the flare-up, the crisis). Temporal medicine focuses equally on the periods of uncertainty that govern whether a person feels safe enough to plan a trip, return to work, or engage fully in daily life.
Clinicians already use fragments of temporal reasoning. But there is no shared framework or system that treats temporal vulnerability as a vital sign. This orientation leads to 3 central clinical dimensions. First, uncertainty itself becomes a therapeutic target. Second, many episodic conditions follow rhythms and recognizing these patterns can guide anticipatory care. Third, resilience in the interepisode period deserves more clinical attention because patients measure progress by whether they feel safe going back to work, traveling, or exercising without fear—not just whether their symptoms are reduced.
Research across disciplines shows the power of explicit temporal reasoning. The following examples share a simple truth: outcomes improve when care anticipates lived rhythms rather than merely reacting to events.
- Inflammatory bowel disease: In a randomized clinical trial, a web-based telemonitoring system for complex inflammatory bowel disease increased remission rates (81% vs 66%-71%) and reduced the number of office visits by enabling proactive remote adjustments before flare-ups occurred.
- Rheumatology: In a machine-learning analysis of activity tracker data, flare-ups were detected with 96% sensitivity and 97% specificity in patients with rheumatoid arthritis and axial spondyloarthritis days before patients reported symptoms.
- Asthma: In a systematic review and meta-analysis, digital inhaler systems were able to alert patients and clinicians when reliever use climbs sharply, prompting timely treatment changes and fewer emergency visits.
- Epilepsy: In a systematic review and meta-analysis, algorithms for seizure forecasting demonstrated moderate discriminative ability (the mean area under the receiver operating characteristic curve was approximately 0.7, indicating the capability to differentiate higher- from lower-risk periods).
The question becomes how to systematize this approach. Not everyone will be convinced that temporal medicine is necessary. Critics note migraine diaries and seizure plans are already used and worry that forecasting might increase anxiety. Yet evidence suggests patients who understand their patterns feel more in control of their episodic illness.
Temporal medicine does not claim to invent timing, it gathers the scattered timing tricks already in use (migraine diaries, seizure-cluster rescue plans, and seasonal asthma advice) and gives them a common scaffold that can scale. Forecasting models remain imperfect, but even modest guidance beats the current void. Heterogeneity is real; seizure clusters, autoimmune flare-ups, and bipolar moods slip in and out of sync. Temporal medicine does not force a single formula; it supplies a flexible structure that adapts to each person’s trajectory and supports shared decision-making.
Technology amplifies rather than replaces judgment. The framework works equally well with artificial intelligence (AI)–powered wearables or a paper calendar asking, “When do you feel most at risk?” The principle is technology agnostic in that a notebook can capture lived patterns as effectively as a smartwatch. What AI and sensors add is scalability to detect subtle shifts continuously across thousands of patients and the ability to deliver timely, individualized forecasts. Artificial intelligence is uniquely suited to operationalize temporal medicine.
Most medical AI focuses on static images or laboratory values, but the real promise lies in analyzing time-series data from wearable devices, inhalers, seizure logs, or biomarkers. Machine-learning models can detect subtle shifts days or weeks before symptoms emerge and generate tailored forecasts. In this way, AI converts temporal medicine from a conceptual lens into a practical clinical tool. The convergence of ubiquitous sensors, AI pattern recognition, and patients’ increasing comfort with self-monitoring has created an unprecedented opportunity to operationalize what clinicians have long intuited: timing matters as much as treatment.
Implementation hurdles of tight clinic schedules, event-based reimbursement, and varying patient appetite for self-tracking are predictable but the payoff is tangible. In patients with inflammatory bowel disease, a telemonitoring system increased remission rates to 81% and cut unscheduled office visits by approximately 30%, which saved roughly €1000 (US $1170) per additional patient in remission compared with standard care and saved approximately €2250 (US $2630) vs telephone follow-up. Remote heart failure monitoring decreases hospital admissions by about one-third, saving $8000 to $10 000 per patient annually.
Making temporal care routine means adding vulnerability windows and recovery curves to the electronic note, expanding metrics to track days free of anticipatory disability, and reimbursing proactive touches such as remote reviews, planning visits, and interpretation of patient-generated time data. Incentives will need to evolve accordingly. Reimbursement that values only crisis visits will always reward reaction. By expanding metrics to include days of anticipatory disability avoided, or recovery time regained, systems can recognize and support the work of preparing patients for life between episodes.
Broader care teams also can help (from psychologists and social workers to occupational therapists) to address temporal flexibility. Equity follows because patient insight is free, whether captured by a smartwatch or a notebook. As AI-driven forecasts mature, regulators must protect privacy without stalling innovation. Temporal medicine offers a framework that brings together practices that already exist, integrates emerging technologies, and helps patients regain a sense of agency in the face of uncertainty.
Such an approach could meaningfully change daily practice for patients. There is no need to wait for perfect tools because much of this thinking already exists in fragments. This framework offers a unifying language for ideas that have until now been scattered. It asks physicians to stop measuring health only by what happens and start measuring by when. Physicians can help shift care from reacting to preparing, from controlling symptoms to restoring confidence, and from managing disease in space to supporting life through time.
Naming temporal medicine matters because it shifts the default of practice. What has been scattered across symptom diaries, rescue plans, and isolated tools becomes a coherent approach that can be taught, measured, and reimbursed. With this lens, uncertainty is no longer invisible; patients’ lived timelines become part of the medical record.”
Full editorial, A Kheder, JAMA 2025.10.6
From the article’s appendix: