Published 2019.12.2
Arguments to track health information over one’s lifetime typically focus on the insights that might be gleaned from recalling information that happened years or decades ago. For those individuals with complicated medical histories or symptoms that defy easy diagnosis, there may be some benefit to tracking symptoms, provider encounters and test results over time. For the rest of us, this may be unnecessary and potentially harmful as others might use this information to charge us higher health insurance premiums or deny us services altogether.
What if tracking some types of health information actually helped us plan for the future? An individual might consider tracking their own health information (symptoms, functional status or quality-of-life) if it could help them better understand their future health needs or help their loved ones anticipate what services might be required in the near or far future. In addition, the information might be valuable to others to help understand the implications of specific healthcare decisions or how specific diagnoses might alter their functional status.
In 1987, Rowe & Kahn defined successful aging as the avoidance of disease and disability. Over time, they updated the definition to include two more components: maintenance of physical and cognitive function and social engagement (specifically, interpersonal relations [contacts and transactions with others, exchange of information, emotional support and direct assistance] and productive activity [i.e., creates societal value]). The World Health Organization’s (WHO) 2015 World Report on Ageing and Health suggests a greater focus on interventions to help older people: Meet their basic needs; learn, grow and make decisions; be mobile; build and maintain relationships; and contribute. Healthcare in America seems to focus nearly exclusively on tracking disability as it affects one’s ability to meet basic needs and decision-making (i.e., activities of daily living [ADLs (getting across a room, dressing, bathing or showering, eating and getting in or out of bed)], instrumental activities of daily living [iADLs (using a telephone, taking medication, handling money, shopping and preparing meals)]), rather than identifying markers that might address opportunities to help individuals more effectively achieve these larger objectives. The WHO’s own Study on Global AGEing and Adult Health (SAGE), tracking individuals in six countries (China, Ghana, India, Mexico, Russian Federation and South Africa) every 4-5 years since 2002, tracks additional metrics (see the Appendix below for a full list of items included in the biennial assessment).
In 2014, Fengyan Tang studied a subset of the University of Michigan Health and Retirement Study (HRS) cohort 65 years of age and older between 2000 & 2008 to determine what patterns of aging might look like. She used six measures to define a successful aging trajectory:
- Chronic diseases (absence of cancer, chronic lung disease, diabetes, heart disease and stroke),
- Activity of daily living (ADL) disability (walking across a room, dressing, bathing or showering, eating, getting in or out of bed, and using the toilet),
- Physical functioning (walking one block; walking several blocks; climbing one flight of stairs; climbing several flights of stairs; lifting or carrying more than 10 pounds; stooping, kneeling or crouching; and pushing or pulling large objects),
- Depressive symptoms,
- Cognitive functioning (memory using two-word list recall tasks [immediate and delayed], working memory using serial 7’s subtraction test, mental status by backward counting, date naming, object naming, and President/Vice-President naming), and
- Active engagement (participation in productive activities like working for pay; volunteering; and helping friends, neighbors or relatives).
Tang identified three groups of individuals: Successful aging (39.6% of males and 32.7% of females), usual aging (38.1% of males and 38.9% of females), and pathologic aging (22.3% of males and 28.3% of females). The figure below outlines the trajectories for all three groups in physical functioning, disability and cognitive functioning:
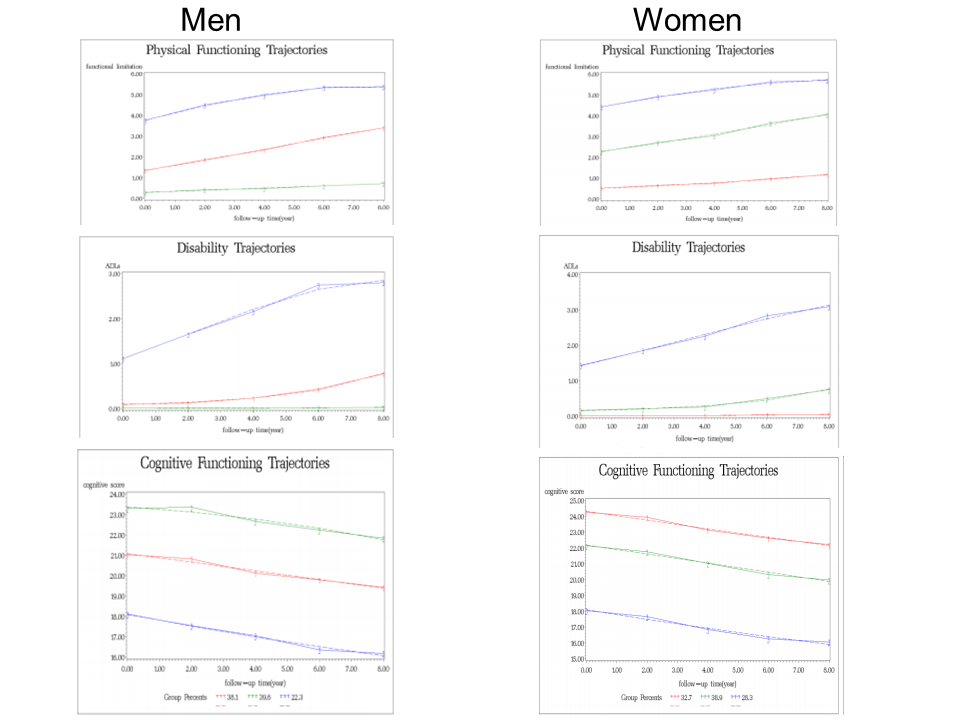
The images on the left refer to the three male groups (green – successful aging, red – usual aging and blue – pathologic aging) and the images to the right refer to the female groups (green – successful aging, red – usual aging and blue – pathologic aging). Images copied from Tang’s 2014 publication in the International Journal of Social Science Studies.
Tang acknowledged several possible limitations including: the absence of subjective assessments, the dimensions may not have been correct and the metrics used to measure the dimensions may not have been correct, and the possibility that transitions might occur among aging trajectories was not considered. In 2014, Xu et al. used the same dataset and included survey responses between 1998 and 2010. They suggested four categories: minimal impairment (29.3%), moderate impairment with increasing cognitive deficit (23.5%), moderate impairment with increasing physical and emotional deficit (24.5%) and significant and increasing impairment (22.6%). The researchers found declines in one dimension (physical, emotional or cognitive functioning) were associated with declines in other dimensions, suggesting considering interventions that combine medical and non-medical interventions.
There are at least three challenges to encouraging individuals to collect this type of information routinely. First, the research studies collected a lot of data. Some of the information can be collected by the individual, other data points require someone (or something) to record the individual’s responses and yet others require a prescription (e.g., laboratory testing, lung function). Even the self-collected information could take more time than many individuals are willing to spend periodically over the rest of their lifetime. Second, it is not clear if people can change their trajectory. If an individual’s trajectory cannot be altered (or may only altered by a small fraction of the population), then the information may promote adverse selection by payers or other entities paying for healthcare or supportive services. Finally, our current processes for tracking longitudinal information do not facilitate trust with the “data collector” or “data storage provider.”
Tracking one’s aging trajectory (after controlling for other factors) would appear to be more efficient than using an age- or ADL-based approach to determine current and future health needs. Existing research has not addressed many salient questions (Can I change my current trajectory by employing different behaviors? How do acute events (e.g., loss of a spouse of family member, injury, surgery) change my trajectory? Do enough baby-boomers care enough about this type to support a start-up in this area?). But if the data collection burden is small enough, individuals may consider collecting at least some of the information on a periodic basis to determine their aging trajectory.
Appendix: Items included in the WHO SAGE study assessments
Stable
- Age,
- Gender,
- Marital status,
- Education,
- Background or ethnic group,
- Religious denomination,
- Where the individual lives and has lived,
- Parents’ occupation and education, and
- Work history and benefits
May require recurring assessments
| Health state descriptions | Anthropometrics and performance tests | Other metrics |
|---|---|---|
| Mobility | Blood pressure | Blood tests |
| Self-care | Height, weight, waist circumference, hip circumference | Risk factors and preventive health behaviors (tobacco and other smoking, alcohol, physical activity, cancer screening) |
| Pain and discomfort | Four-meter timed walk (normal and rapid) | Health care utilization (inpatient, outpatient and care at home) |
| Cognition | Vision test (near and distance) | Social cohesion |
| Interpersonal activities | Grip strength | Impact of caregiving |
| Sleep and energy | Verbal recall (immediate and delayed) | |
| Affect | Digit span (digits forward and backward) | |
| Vision | Verbal fluency | |
| Chronic conditions (arthritis, stroke, angina, diabetes, chronic lung disease, asthma, depression, hypertension, cataracts, oral health and injuries) | Lung function | |
| Functioning assessment | ||
| Subjective well-being and quality of life (including day reconstruction) |